Recently, An international academic journal with an impact factor of 5.246, Frontiers in Molecular Biosciences, published a paper named Methylation of SDC2/TFPI2 and Its Diagnostic based on the prototype of Elchang Kang products Value in Colorectal Tumorous Lesions.
The Diagnostic value of SDC2/TFPI2 methylation in colorectal tumor Lesions
This paper has aroused the attention of many cancer detection researchers. The paper compares the differences between the double-target stool DNA detection and other detection methods in detail, and once again proves that: Among the many early screening methods for faecal DNA colorectal cancer, compared with the traditional SDC2 single-target detection method, the double-target faecal DNA tumor detection technology of Alchangkang ® has higher sensitivity and specificity indicators, and can also detect precancerous lesions of colorectal cancer earlier.
Colorectal cancer (CRC) affects millions of people worldwide. It is unique in that it progresses slowly, making it preventable and often curable, and early detection can significantly reduce mortality. Colonoscopy plus pathological testing is the gold standard for colorectal cancer diagnosis, but due to the invasive and complex nature of the bowel preparation process, compliance is low in people at average risk.
Traditional detection methods fecal occult blood test (FOBT) and fecal immunochemical test (FIT) are non-invasive, but their sensitivity is insufficient, especially for stage I colorectal cancer and advanced adenomas.
Limitations of traditional SDC2 single-target detection
Abnormal DNA methylation can occur at a very early stage, and so far, several colorectal cancer methylation biomarkers have been identified, including SDC2, NDRG4, BMP3, VIM, SFRP2, and SEPT9, but the sensitivity of individual markers is usually less than 90%. SDC2 has been identified as a potential biomarker for colorectal cancer. Abnormal methylation in SDC2 CpG islands has been found in tissues, blood and feces.
A study based on fecal samples from China showed that the sensitivity and specificity of SDC2 for colorectal cancer were 81.1% and 93.3%, respectively. The Korean researchers enriched SDC2 using LTE-q methylation-specific PCR (MSP) method, which requires two rounds of PCR, that is, first one-way linear amplification of the target DNA, and then MSP analysis of the target region, with 90.0% sensitivity and 90.9% specificity for colorectal cancer detection.
New method: double target detection with better performance
Figure 1 shows the comparison of sensitivity of different assesses (SDC2 alone, TFPI2 alone, and SDC2/TFPI2 combined) for lesions at different sites of colorectal cancer.
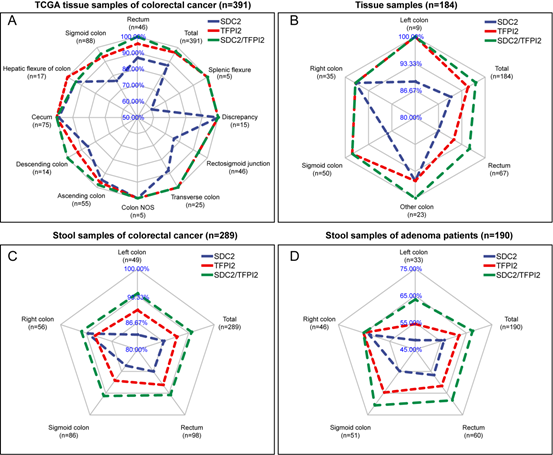
Figure 1. Comparison of sensitivity of SDC2, TFPI2 and SDC2/TFPI2 in detecting colorectal cancer at different sites.
The blue line represents the detection sensitivity of SDC2, the red line represents the detection sensitivity of TFPI2, and the green line represents the combined detection sensitivity of SDC2/TFPI2.
Data result:
Based on the above four data sets, it was found that the sensitivity of SDC2/TFPI2 combined detection was significantly higher than that of SDC2 single gene detection. TFPI2 can improve the detection sensitivity of SDC2, especially for left colon cancer, rectal cancer and sigmoid cancer. Tissue samples and stool samples showed the same trend.
Experimental conclusions:
Compared with the single-target SDC2 detection method, the two-target SDC2+TFPI2 combined detection of Alchangkang ® has more advantages.
New findings: Dual target detection, better detection of early adenomas
With colonoscopy as the gold standard and MSP result as the evaluation index, the diagnostic performance of SDC2, TFPI2 and SDC2/TFPI2 in fecal samples was evaluated by ROC curve analysis (Figure 2 and Table 1). Figure 2 (A-C) shows the ROC curve based on Ct value, and Figure 2 (D-F) shows the ROC curve based on ML value.
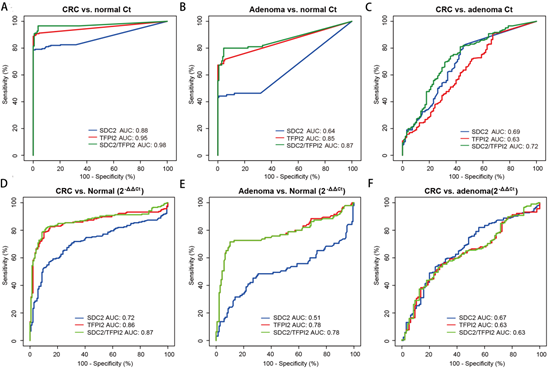
Figure 2. Diagnostic performance of methylation-specific PCR targeting SDC2, TFPI2, and SDC2/TFPI2 in fecal samples.
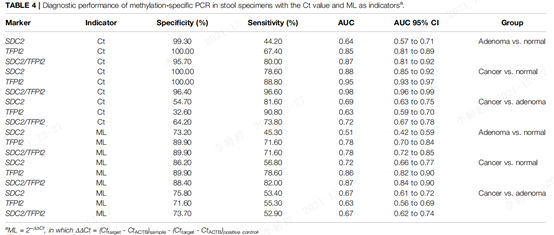
Table 1. Diagnostic performance of methylation-specific PCR of fecal specimens based on Ct values and ML
Data result:
Ct-based ROC analysis (Figure 2 (A) and Table 1) showed that the AUC value of SDC2/TFPI2 differentiating colorectal cancer vs adenoma group was 0.72, which was lower than that of colorectal cancer vs normal group and adenoma vs normal group.
Experimental conclusions:
Alchangkang ® dual target SDC2+TFPI2 combined detection can better detect early adenomas.
In recent years, Emison Life Technology has developed a series of products for the early diagnosis of multiple high-incidence malignant tumors, including digestive system tumors (bowel cancer, stomach cancer, liver cancer, esophageal cancer, pancreatic cancer), gynecological tumors (cervical cancer, endometrial cancer), urinary system tumors (bladder cancer), and pan-cancer species.
With outstanding product performance, Emison Life Technology has won four rounds of hundreds of millions of yuan of financing and strong support from governments at all levels, and has won various honorary titles.

Behind the excellent clinical performance indicators and technical platform is the unswerving heart of doctors, and escorting the life and health of the public is the persistent pursuit of Emison Life Technology!
Cancer early screening, fight cancer
Emison Life Technology, keep moving!
About Emison Life Technologies
Emison Life Technology focuses on early diagnosis and screening of high-incidence malignant tumors, providing genetic detection services for high-incidence malignant tumors such as digestive system tumors (bowel cancer, stomach cancer, liver cancer, esophageal cancer, pancreatic cancer), gynecological tumors (cervical cancer, endometrial cancer), urinary system tumors (bladder cancer), and pan-cancer.
The company has been based on local development for many years, the core technology is independent and controllable, and the core members come from well-known universities in the United States, Japan, Italy and other places and domestic double first-class universities. After several years of accumulation, a set of diversified product detection system with methylation navigation technology as the core was established. After tens of thousands of clinical sample verification, screening sensitivity and specificity have reached the industry-leading level! The company has applied for 55 patents in total, published more than 10 cutting-edge research results, and has become one of the few multi-cancer screening service providers in the world and an early-diagnosis and early-screening enterprise with external technology authorization License-out.
