Retrospective content:
On December 21, last year, an international academic journal with an impact factor of 5.246, Frontiers in Molecular Biosciences, published a medical paper based on the prototype of Alchangkang products, which was recognized by many experts.

Previous studies have shown the potential of double-target fecal DNA testing for colorectal cancer (CRC), and this article describes the comprehensive performance of ALchangkang ® in a multicenter clinical trial.
Recently, THE results OF the multicenter clinical trial of Elchangkang ® were published in THE JOURNAL OF MOLECULAR DIAGNOSTICS with an impact factor of 5.568. It is called 'Evaluating the Clinical Performance of a Dual-Target Stool DNA Test for Colorectal Cancer Detection.'

Evaluation of Clinical Performance of Double-target Fecal DNA Testing for Colorectal Cancer
A total of 1164 subjects from three independent hospitals, namely Peking Union Medical College Hospital, Zhongnan Hospital of Wuhan University and the First Affiliated Hospital of Anhui Medical University, were included in this study using a double-blind case-control study, including 320 patients with CRC, 148 with adenoma, 396 with interfering diseases and 300 with healthy control group (figure below). The main indexes to evaluate the experimental results include sensitivity, specificity and accuracy.
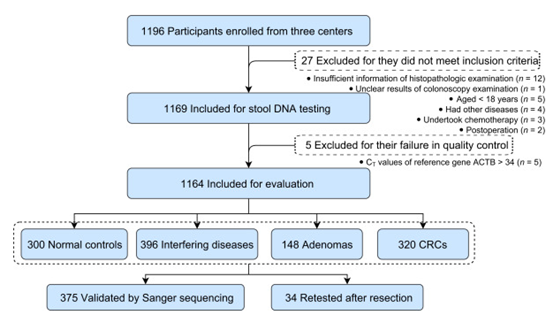
How:
All 1164 samples were tested by methylation-specific PCR (MSP).
Sanger sequencing was performed in 375 samples.
The methylation-specific PCR test was performed again in 34 participants whose tumors had been removed.
Experimental results:
[CRC detection performance] The sensitivity of CRC detection was 95.31%, the specificity in normal controls was 96.67%, and the accuracy was 90.29%.
[Early detection rate performance] Sensitivity to advanced adenoma was 63.16%.
[Anti-interference performance] When combined with interference disease, the specificity was 88.39%.
【Sanger sequencing verification performance 】 The average accuracy of methylation status of 375 samples verified by Sanger sequencing was 99.62%.
[Post-operative validation performance] 34 participants were tested for methylation a second time after surgical resection, with an accuracy of 94.12%.
First, [overall performance of CRC detection] The detection sensitivity and specificity are high, and the early detection rate is high
MSP was detected in 320 CRC samples and 300 normal control samples, and compared with pathological results.
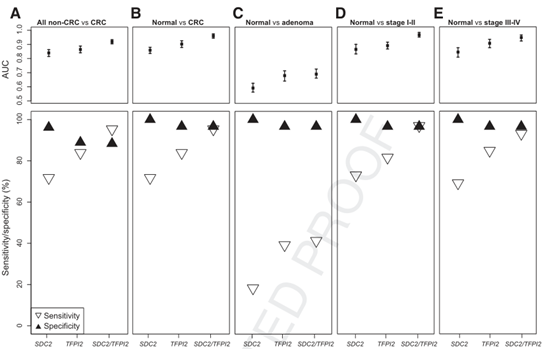
Experimental results:
The sensitivity of CRC was 95.31% (95%CI: 93.00%-97.63%), and the specificity in healthy controls was 96.67% (95%CI: 94.64%-98.70%).
The mean sensitivity of early (Ⅰ and Ⅱ)CRC detection was 96.93%.
Experimental conclusions:
Alchangkang ® dual target DNA assay has high sensitivity and specificity;
Elchangkang ® dual target DNA test enables more accurate detection of early adenomas.
Second, [Anti-interference performance] : Elchangkang ® dual target DNA detection has a good recognition of interference diseases
The study recruited 396 participants with common digestive disorders classified as interfering disorders. The positive detection rate of interference disease was 6.70% (n=27), and the specificity was 93.15% (n=367).

Results: The sensitivity and specificity of CRC detection were 95.31% and 96.67%, respectively, and the accuracy was 90.29%. When combined with interference disease, the specificity was 88.39%.
Conclusion: Elchangkang ® dual target DNA detection has a good identification of interfering diseases.
3. [Compared with Sanger sequencing results] the consistency was 99.62%
The methylation status of SDC2 and TFPI2 in 375 samples was verified by Sanger sequencing. By sequencing MSP products, it was observed that the consistency between MSP results and sequencing results of SDC2 was 100% (kappa =1.00), and that between MSP results and sequencing results of TFPI2 was 98% (kappa =0.98), with an average accuracy of 99.62%.
Data result:
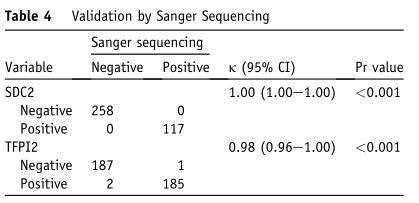
Experimental conclusion: Alchangkang ® double-target DNA detection technique can detect abnormal gene methylation very accurately.
Iv. [Postoperative patient follow-up] Postoperative detection accuracy rate 94.12%
The 34 patients were followed up and their stool samples were tested a second time within 2 months after surgery, and the results were as follows
|
| Before resected |
|
| Positive | Negative |
After resected | Positive | 2 | 0 |
Negative | 28 | 4 |
Data results: 4 cases of CRC confirmed as false negative before surgery were tested negative again after surgery, and the other 28 cases of CRC with positive MSP were all negative after surgery. The accuracy was 94.12%(32/34), except for 2 CRCS that did not match expectations.
Conclusion: The double-target DNA detection technique of Elchangkang ® can be used well for postoperative monitoring of patients.
In recent years, Emison Life Technology has developed a series of products for the early diagnosis of multiple high-incidence malignant tumors, including digestive system tumors (bowel cancer, stomach cancer, liver cancer, esophageal cancer, pancreatic cancer), gynecological tumors (cervical cancer, endometrial cancer), urinary system tumors (bladder cancer), and pan-cancer species.
With outstanding product performance, Emison Life Technology has won four rounds of hundreds of millions of yuan of financing and strong support from governments at all levels, and has won various honorary titles.

Behind the excellent clinical performance indicators and technical platform is the unswerving heart of doctors, and escorting the life and health of the public is the persistent pursuit of Emison Life Technology!
Cancer early screening, beat cancer
Emison Life Technology, keep moving!
About Emison Life Technologies
Emison Life Technology focuses on early diagnosis and screening of high-incidence malignant tumors, providing genetic detection services for high-incidence malignant tumors such as digestive system tumors (bowel cancer, stomach cancer, liver cancer, esophageal cancer, pancreatic cancer), gynecological tumors (cervical cancer, endometrial cancer), urinary system tumors (bladder cancer), and pan-cancer.
The company has been based on local development for many years, the core technology is independent and controllable, and the core members come from well-known universities in the United States, Japan, Italy and other places and domestic double first-class universities. After several years of accumulation, a set of diversified product detection system with methylation navigation technology as the core was established. After tens of thousands of clinical sample verification, screening sensitivity and specificity have reached the industry-leading level! The company has applied for 55 patents in total, published more than 10 cutting-edge research results, and has become one of the few multi-cancer screening service providers in the world and an early-diagnosis and early-screening enterprise with external technology authorization License-out.